

Natural water is not usually a chemically pure substance. It contains dissolved salts, minerals, organic compounds and gases. These substances can occur in varying concentrations depending on the source of the water. Dissolved salts exist as ions and can be removed from the water using an ion exchange process or adsorption (= binding to solid state).
Ions can carry one or more charges. Usually, they have 1,2 or 3 charges
(e.g. +, ++, +++). Moreover, ions can also consist of only a single atom (e.g. H+) or a combination of several indissolubly linked atoms, forming a molecule (e.g. CO3--).
The charge of ions in the water always must be zero. This means the number of positive and negative ions is the same.
Ion exchange is a very powerful method to remove impurities, residues and contaminants from water.
For the ion exchange, substances are used that have a surface property allowing ions to adhere very well (= so-called ion exchangers). These ion exchangers are loaded with positively charged hydrogen ions H+ and/or negatively charged hydroxide ions OH-.
These ions have a low charge (+) (-).
The higher the charge and the smaller the radius of an ion, the more the ion is bound to the ion exchanger.

If the water to be treated is now passed through an ion exchanger with positive and negatively charged ions, all positively charged cations (+) in the water are replaced with positively charged hydrogen ions (H+) and all negatively charged anions (-) in the water are replaced with negatively charged OH ions (OH-). This means that the ion exchanger repels all hydrogen and hydroxide ions and picks up the positive and negative ions from the water. The H+ and OH- ions repelled by the ion exchanger now combine to ultrapure, residue-free water outside of the ion exchanger.
This process takes place until the ion exchanger no longer can give off any H+ or OH- Ions.
Deionised water is also called fully demineralised water.
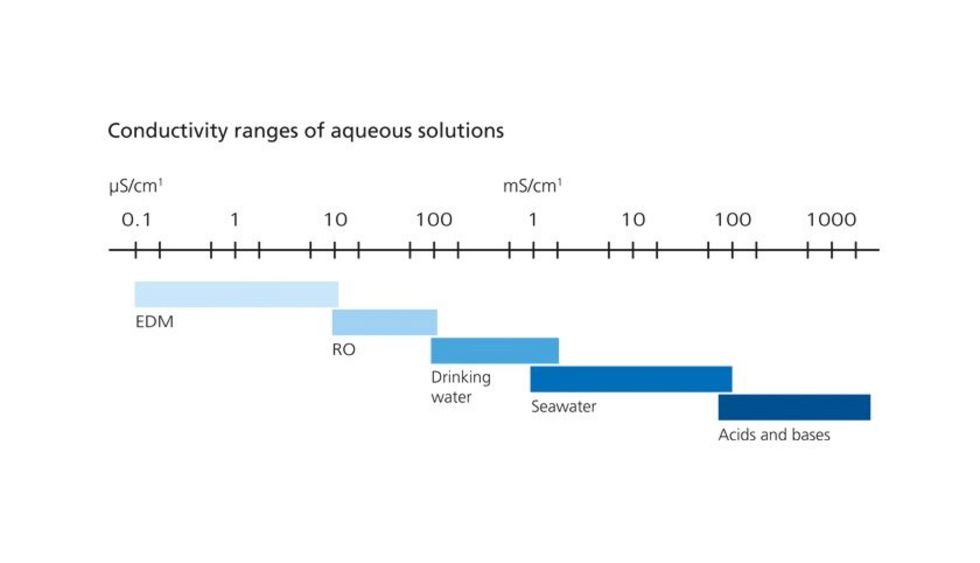
The purity of demineralised water is expressed through its conductivity.
In solutions, the current is carried by cations and anions = electrical conductivity. Cations and anions are thus the mobile charge carriers.
This conductivity is specified as a conductance value in µs/cm1.
The purer the water, the lower the conductance value. Ultrapure water, for example, has a very low conductance value of <0.1 µs/cm.
During partial desalination (= selective ion exchange), the ion exchanger is loaded only with H+ ions or OH- ions. This means that the ion exchanger can exchange only positively or negatively charged ions.
Partial desalination usually is used for the removal of hydrogen carbonate salts. This means only the positively charged cations CA++ and Mg++ from the carbonate hardness elements calcium carbonate and magnesium carbonate are replaced with hydrogen ions H+.
The CaCO3 carbonate hardness thus results in carbon dioxide.
(H+) + (HCO3) = H2CO3
In contrast to fully demineralized water, all other salts in the water remain in place. A partial desalination is therefore only useful if simple water softening is sufficient for the application, for example (as described in the process above).
It is also possible to selectively load the ion exchanger to use adsorption to remove only specific heavy metals (e.g. lead, cadmium or mercury) but also anions such as nitrate and sulfate.
The choice of desalination process, as well as the degree of desalination arise from the desired purity of water as well as the procedures and the operational and economic conditions.

All dissolved salts are removed from the water with full demineralization.
The ion exchanger is loaded with positively charged H+ as well as negatively charged OH- ions. If the water to be treated is now passed through the ion exchanger, all positively and negatively charged ions are replaced or adsorbed (= bound to solid state).
As already described in the section "The principle of ion exchange", ultrapure water is now the result of the emission of H+ and OH-.
(H+) + (OH-) = H2O
As opposed to distilled water, fully demineralized water is not acidic and has decisive advantages compared to osmosis water due to the removal of carbon dioxide.
• Protection against corrosion and insoluble deposits by the removal of chlorides, sulfates and nitrates from the water.
• Depending on the quality of the incoming water, the electrical conductivity drops down to < 0.1 µs/cm1 (TREF25).
• The pH value is in the neutral range because carbon dioxide and silicic acid is removed.
• The low conductance value and neutral pH remain constant and thus guarantee long service life of your machines.
• Dissolved inorganic ions are easily removed.
The resistance after treatment is 18.2 MΩ (at 25 °C) with a TOC value of <1 ppb.
