

Osmosis is based on a natural physical process by which plants draw moisture out of the ground with their root cells.
Our own metabolic processes in cells is based on osmosis as well.
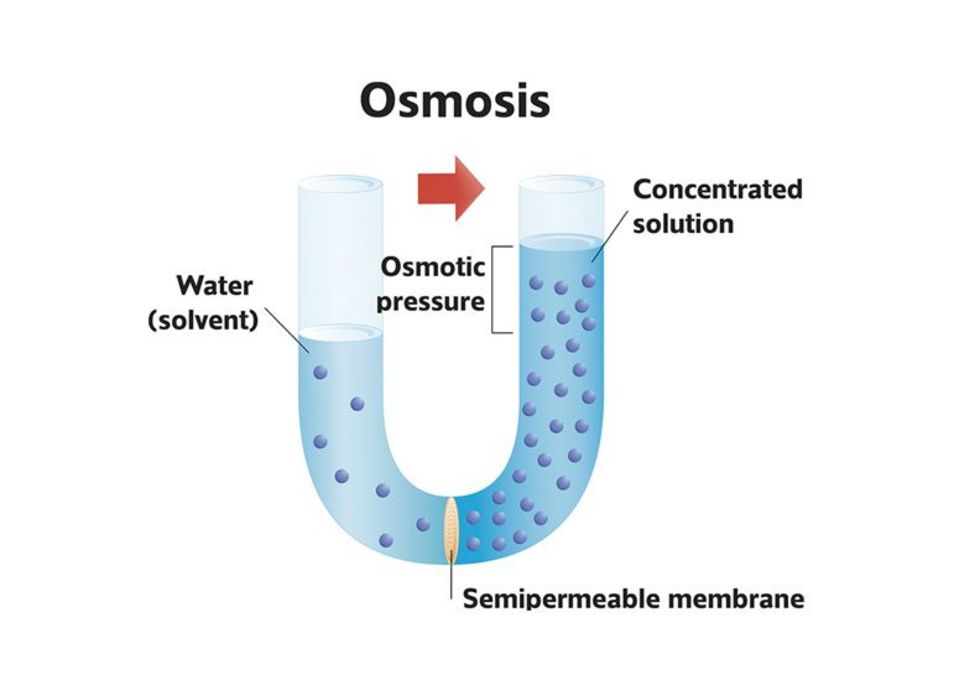
Osmosis is the targeted diffusion of water through a semipermeable membrane or diaphragm.
Semi-permeable membranes have special properties.
They are permeable to water molecules, while larger molecules, such as salt particles, cannot pass the membrane.

Example: Salt solution
Osmosis balances the concentration of solutions separated by a semi-permeable wall.
Water tries to reach a balanced solution on both sides of the membrane.
This happens until the concentration on both sides is the same or the water pressure of the salty side equals the osmotic pressure.
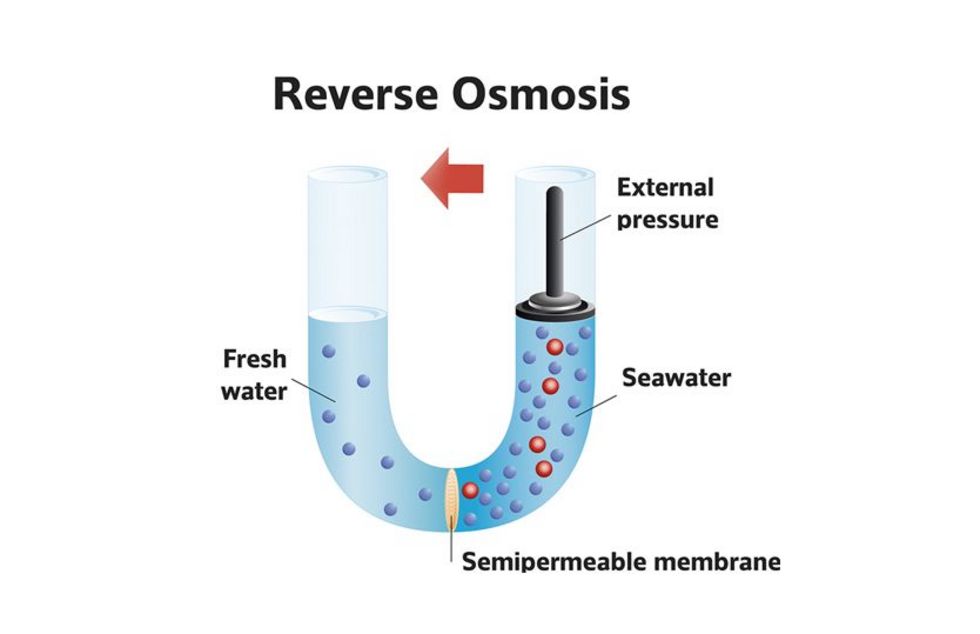
Our salt solution is subjected to pressure and pressed against the semipermeable membrane.
Applied pressure > osmotic pressure!
This makes water molecules move from the higher concentrated to the less concentrated solution* - the reverse to osmosis.
Example: Salt solution
The concentration of the two sides is not balanced by the applied pressure.
* but only water molecules and no other molecules.
Filtration means separation only in a physical and mechanical way.
During the filtering process, mixtures (suspensions) are mechanically separated from liquids containing solids (particles or molecules).
During water treatment, the optimum filtration processes or a combination of several methods is chosen depending on the quality of the raw water as well as the specifications for purity after filtration.
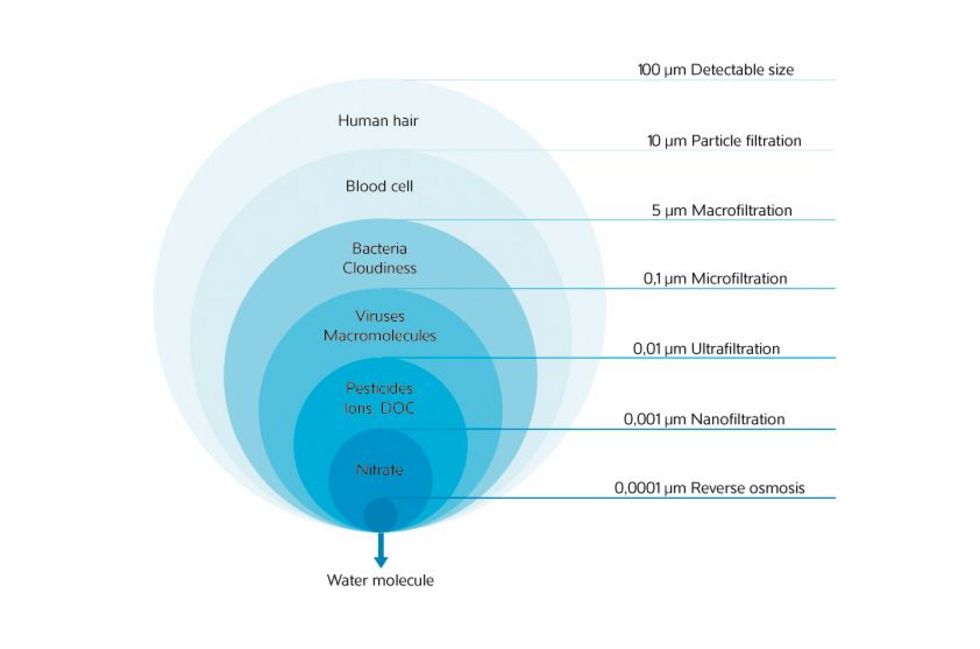
Membrane filtration is a dynamic filtering process. The flow direction of the liquid or gas is here not vertically through the filter area but horizontally through membranes. By selecting a filter pore size of a membrane, the fineness of filtration, i.e., the size of the particles to be removed, can be determined.
Picture left side: Filtration fineness of the described procedures
This is used mainly in the food industry and serves as filtration technology for fluids with high murky contents (such as fruit juice, wine, etc.) but finds use in dialysis as well.
The material of the filter surface in microfiltration may be - depending on the application area - made from plastic, textile fabric or stainless steel. The filter pore size of this technique is > 0.1 µm.
Microfiltration is used, for example, to separate oil-water emulsions, biotechnology applications, or to separate colloidal oxides or hydroxides.
The filter pore size of ultrafiltration is 0.1 to 0.01 µm. Ultrafiltration is used for such applications as separating proteins, cold sterilization in the pharmacy, or for metal recovery and wastewater cleaning in the field of metallurgy.
Nanofiltration is a pressure-driven membrane processes. In contrast to reverse osmosis, nanofiltration uses lower pressures and filters with larger pore sizes. The particle size, which is retained in nanofiltration, is the size of single and divalent heavy metal ions. Nanofiltration is used, for example, for softening water and the removal of heavy metals during drinking water treatments.
Reverse osmosis or also is a pressure-driven membrane process in which pressure reverses the natural osmosis process.
It is used to treat drinking and process water, aquarium water, to produce fruit juice concentrates, and in the treatment of wastewater.

The reverse osmosis membrane technology (reverse osmos = RO) is used for the separation of salts and ions from predominantly aqueous solutions.
No addition of chemicals
Low energy consumption
Easy to use
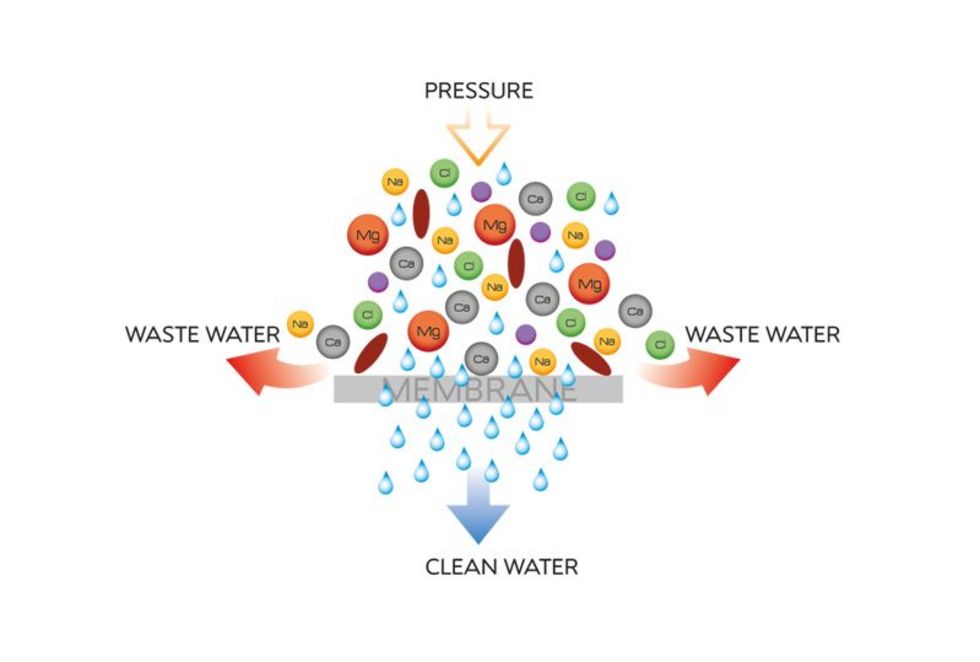
A semi-permeable membrane is used in reverse osmosis for the separation of the individual components of an aqueous medium (e.g. water).
This membrane has the property that the aqueous component (pure water = permeate) can pass through the membrane, while other components such as suspended solids, salts, etc. (= concentrate) are for the most part held back and disposed of through the wastewater drain.
The membrane thus acts similar to a filter which only allows pure water to pass through and holds back larger molecules than the pure water molecule; however this membrane, in contrast to the filter (e.g. microfiltration, ultrafiltration) has no real pores.
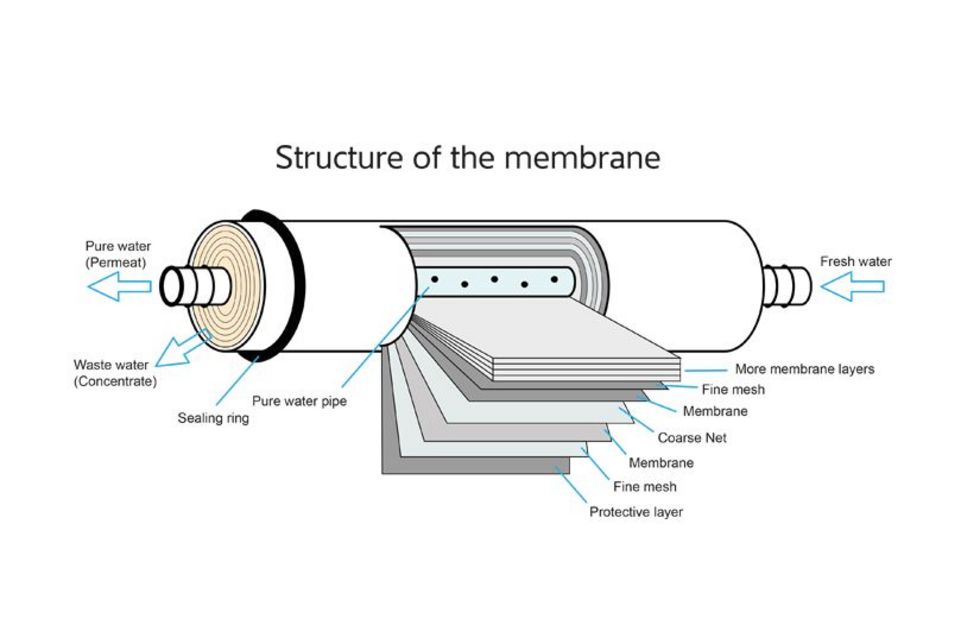
The separation of the components is done through a diffusion membrane with nanofiltration as well as reverse osmosis.
The pressure necessary to push the medium through a diffusion membrane is quite higher than with microfiltration or ultrafiltration. This does reduce productivity but the filtration efficiency greatly increased.
The retention of calcium (hardness) is > 99%. The filtration efficiency varies with the quality of the membrane and the quality of the aqueous medium (feed water).
To achieve a surface that is as large as possible with the smallest volume, the individual layers of the membrane are closely wrapped. There are numerous types and designs.
In the EASYRO® reverse osmosis system, we use tube-shaped low pressure high performance membranes. The membrane technology used here is state of the art. Only industrial components are used, which cannot be compared with conventional
membranes in any way.

The permanent diffusion work of a membrane makes deposits on the individual membrane layers unavoidable. A pre-filtration before the reverse osmosis system removes coarser pollution particles such as suspended solids and colloids but does not prevent a regular cleaning rinsing of the membranes.
A membrane absorbs water-insoluble salts during when doing their job. In case of inorganic salts such as calcium carbonate and barium sulfate, precipitation (scaling) may easily occur due to supersaturation. This means the membrane may become blocked. The diffusion properties of the membrane layers continues to diminish and the service life of the membrane is reduced enormously.
The yield of permeate can no longer be guaranteed and the pressure in the system rises.
In the worst case, the pressure exceeds the permissible value and damages the system.
Regular maintenance of the water treatment system is thus very important. During a cleaning flush, any deposits on the membrane layers are removed and discharged with the wastewater. The membranes then are again free of residues and fully operational.
We gladly undertake the regular maintenance of your EASYRO® system for you. Conclude a service and maintenance contract with us and we will automatically remind you of overdue maintenance and make an appointment for you.